The potential of E-fuels as future fuels
DOI 10.12910/EAI2021-022
by Rosanna Viscardi, Claudia Bassano, Paolo Deiana Production - Storage and Use of Energy Division, Energy Storage, Batteries and Technologies for Hydrogen Production and Utilization Laboratory. Giuseppe Nigliaccio - Technologies for Districts Urban and Industrial Laboratory
E-fuels are renewable, climate-friendly and can be used as energy carriers and feedstock; they are the most sustainable solution to meet the energy demand of a growing global economy and will play an important role in an optimized future energy system. Technologies for production of this kind of fuels are proven and tested in many installations worldwide, but processes have not yet been scaled up to industrial scale and like any emerging technology, production costs for e-fuels are currently high. In order to reduce costs in an effort to penetrate the market, it is strategic, together with the development of technological aspects, also the creation of industrial synergies. Currently ENEA there is a multidisciplinary technological know-how with skills that cover the complete e-fuels value chain; future development projects may involve activities on laboratory scale and on prototype and pilot plant scale covering different TRL value. Process and predictive analyses, technical economic and environmental feasibility studies will also be carried out. ENEA, as proposals´ leader within funding programs, develops various applied research projects by integrating lines of activity that aggregate industrial partners with various core business, but which can find interest in the common development of technology.
Gli e-fuel sono rinnovabili, rispettosi del clima e possono essere utilizzati come vettori energetici e materie prime; sono la soluzione più sostenibile per soddisfare la domanda energetica di un'economia globale in crescita e giocheranno un ruolo importante in un futuro sistema energetico ottimizzato. Le tecnologie per la produzione di questo tipo di combustibili sono provate e testate in molte installazioni in tutto il mondo, ma i processi non sono ancora stati portati su scala industriale e, come ogni tecnologia emergente, i costi di produzione degli e-fuel sono attualmente elevati. Per ridurre i costi nel tentativo di penetrare nel mercato, è strategico, insieme allo sviluppo degli aspetti tecnologici, anche la creazione di sinergie industriali. Attualmente ENEA dispone di un know-how tecnologico multidisciplinare con competenze che coprono l'intera catena del valore degli e-fuel; i futuri progetti di sviluppo possono comprendere attività su scala di laboratorio e su scala di prototipi e impianti pilota che coprono diversi valori di TRL. Verranno inoltre svolte analisi di processo e predittive, studi di fattibilità tecnica economica e ambientale. L'ENEA, in qualità di capofila delle proposte all'interno dei programmi di finanziamento, sviluppa diversi progetti di ricerca applicata integrando linee di attività che aggregano partner industriali con diversi core business, ma che possono trovare interesse nello sviluppo comune della tecnologia.
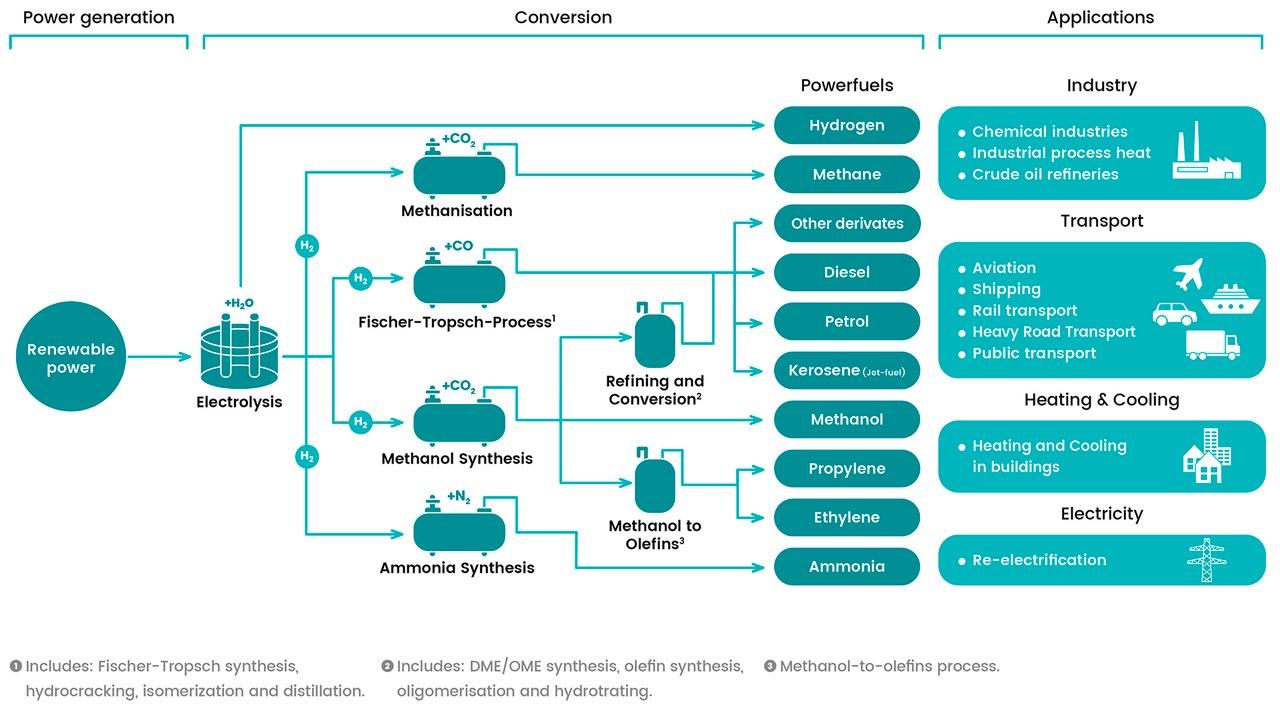
E-Fuels, or electrofuels, or Powerfuels, refers to any technology which converts (renewable) electrical power to a gaseous or liquid energy carrier. There are different taxonomies that refer to e-fuels: in the REDII the Commission uses the terminology ‘Renewable liquid and gaseous transport Fuels of Non-Biological Origin’ (RFNBO) and in the “A hydrogen strategy for a climate-neutral Europe” the Hydrogen-derived renewable synthetic fuels refer to a variety of gaseous and liquid fuels on the basis of hydrogen and carbon where the hydrogen should be renewable. Renewable fuels can be promoted most effectively if they can be easily distinguished from more polluting energy sources. Therefore, the Commission will work to introduce a comprehensive terminology and a European certification system covering all fuels renewable. As regards to e-fuel production pathways, several conversion routes are identified. Using the electrolysis process electrical power is converted to hydrogen thus allowing decoupling the energy from the electricity sector for use in other sectors. Hydrogen can then be used directly as a fuel, or alternatively reacted with either CO2 or nitrogen to produce a range of different gaseous or liquid fuels. We can distinguish: power-to-liquids (Fischer-Tropsch, methanol, DME, ammonia and other synthesis liquid) and power-to-gas (hydrogen, methane,). In all pathways the outcome is carbon neutral, provided that the electricity used comes from renewable sources.
E-fuels offer many advantages: being currently able to bring carbon emission reduction, being carbon neutral, making use of the existing infrastructure and even more importantly of the existing vehicle fleet [1]. They feature a significant energy content per mass, and can be moved, stored in liquid or gaseous form, in the long term, without energy losses. They provide energy security and reliability to energy system. The storability and portability of these fuels allow to stock necessary volumes to be used in case of black out or any other supply issue with other energy carriers.
Their production and deployment must be stepped up to deliver their full potential to fight against climate change.
The overall energy efficiency of various e-fuels production pathways is about 50%, because of the energy used in the course of the synthesis process. The production costs of liquid e-fuels (based on the Fischer-Tropsch production pathway) is estimated at 2050 between a cost of approximately 5.2 to 9.6 €ct/kWh (0.5 and 0.9 €2018/lliquid). According to Irena (2019) ammonia production cost is in the range of 0.5 -0.6 $2018/kgNH3. Methanol and DME production costs may lie in the order of 100-210 €2030/MWhmethanol and 100-310 €2030/MWhDME in the future [2]. Finally, Synthetic natural gas production cost are in the range of 8-60 €2017/MWhSNG [3]
Like any emerging technology, production costs for e-fuels are currently high. Economies of scale are expected with the development and deployment of these solution.
With regard to R&D challenge in the synthesis step, major challenges are the direct utilisation of CO2, with research concerning catalyst step, catalyst behaviour under flexible and dynamic operation.
Additional research concerning the influence of reactor and process design choices on the dynamics and the determination of the dynamic plant behaviour should be given more attention, as well as techno-economic guidance to improve design choices. These challenges could be different depends on the PtL product.
Research and development activities in ENEA
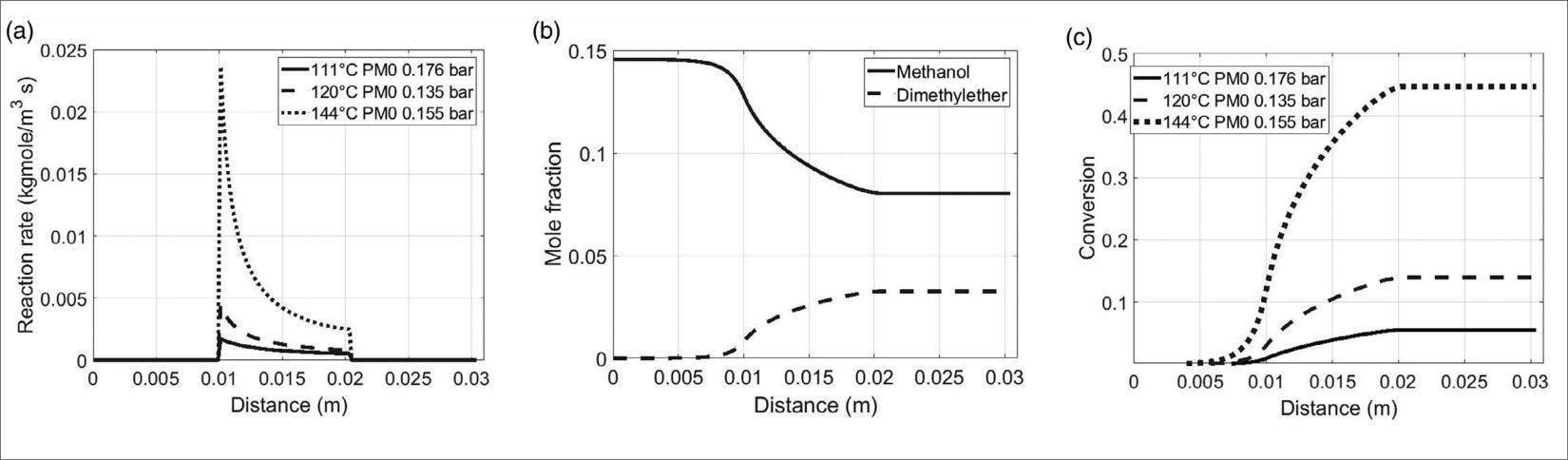
The major obstacle in the transformation of CO2 to e-fuels consists in the inertness of the CO2 molecule and the associated substantial energy required for the carbon reduction. Additionally, checking the selectivity of the desired product is not trivial due to the multiple competing reactions involved, like reverse water–gas shift (RWGS) or FT. Therefore, the research of efficient and highly selective catalysts is crucial to improve process sustainability [5]. This last point is very important since the above mentioned inertness of CO2 requires the utilization of expensive hydrogen as a co-reagent to activate CO2. Therefore, any loss of selectivity implies the consumption of avoidable H2 that hinders the final economic process viability. Since 2014 by the National Program on Electric System Research funded by the Italian Ministry of Economic Development, at the ENEA lab experimental tests have been carried out in order to optimize the CO2 hydrogenation to methanol/DME. Our laboratory was involved in the research of an appropriate catalyst with suitable acid strength, pore size, morphology, active temperature range, toxicity and coking resistance that provided a useful tool to lead DME direct synthesis by CO2 hydrogenation. In addition, it was necessary to find a material having good resistance to the water produced from CO2 hydrogenation or CO2 rich syngas because water is believed to block the active sites for methanol consumption during one-step synthesis of DME [6]. For these reasons, in collaboration with University of Parma, we have successfully developed and tested a new class of SO3H-functionalized materials that has been proposed for the first time as efficient catalysts to perform the methanol dehydration process to DME [7].
These materials were very active, selective and stable catalysts for methanol to DME transformation. Moreover, their catalytic activity and water resistance were better than that of reference commercial catalyst for this process. These materials will be tested as acid part of the bifunctional catalyst for DME direct synthesis in the experimental facility that we are developing in the next months. The purpose of this highly versatile experimental apparatus will be to find the most economical route towards the synthesis of these fuels from CO2 and green hydrogen. To reach this objective:
- fundamental mechanistic understanding,
- the development of efficient multifunctional catalysts,
- rigorous testing and characterization of the obtained fuels, and
- the design of the most adequate process layout have to go hand in hand.
We strongly believe that the lack of ex situ characterization of the spent catalyst component needs to be addressed by the scientific community to achieve a fundamental mechanistic understanding, an essential requisite for catalyst development.
Projects and perspectives
Gaseous and liquid fuels and feedstocks will still play an important role in an optimized future energy system. From today’s perspective, energy carriers with high energy density as well as specific raw materials have great difficulties being replaced. This can be seen especially in both the chemical and transport sectors. E-fuels are the most sustainable solution to meet the energy demand of a growing global economy, even in the future. Technological progress and cost reductions in wind and solar power, as well as electrolysers, have been significant in recent years. Therefore, even from a cost perspective, there has been an increasing amount of interest in creating a market for those fuels. Combining these factors will mean that E-fuels can offer new opportunities. These can be shortly summarized in few points:
- Complement to direct electrification in all sectors: E-fuels provide an opportunity to use renewable fuels in all sectors and in nearly all available applications. They complement the direct use of renewable energies, specially where direct electrification is not possible or economical.
- Use of existing infrastructure: E-fuels can be integrated completely or partly into the existing gaseous and liquid fuel infrastructure.
- Energy security and diversification: E-fuels contribute to energy security and energy stability.
- Sustainability and costs: E-fuels can be more efficient and less resource-intensive than biomass products, but are currently more expensive.
- Global commodity and new markets: E-fuels could become a tradeable global commodity. Countries with low natural resources but good solar and wind conditions, will get the chance to improve their own energy supply and become a new player in the global energy market.
- Technological innovation progress and local benefits such as new employment in industry and in research and development.
- Storage: e-fuels can also be considered a valid system for the seasonal storage of the surplus of production from RES. Compared to batteries, therefore, they have a different usage target in terms of the amount of energy that can be stored and times of usage.
Currently in ENEA there is a multidisciplinary technological know-how with skills that cover the complete e-fuels value chain. Future development projects may involve activities on laboratory scale (catalyst synthesis and test) and on prototype and pilot plant scale covering different TRL value. In this contest, process and predictive analyses, technical economic and environmental feasibility studies will be carried out.
In order to reduce costs in an effort to penetrate the market, it is strategic, together with the development of technological aspects, also the creation of industrial synergies.
ENEA, as proposals´ leader within funding programs, develops various applied research projects by integrating lines of activity that aggregate industrial partners with various core business, but which can find interest in the common development of technology.
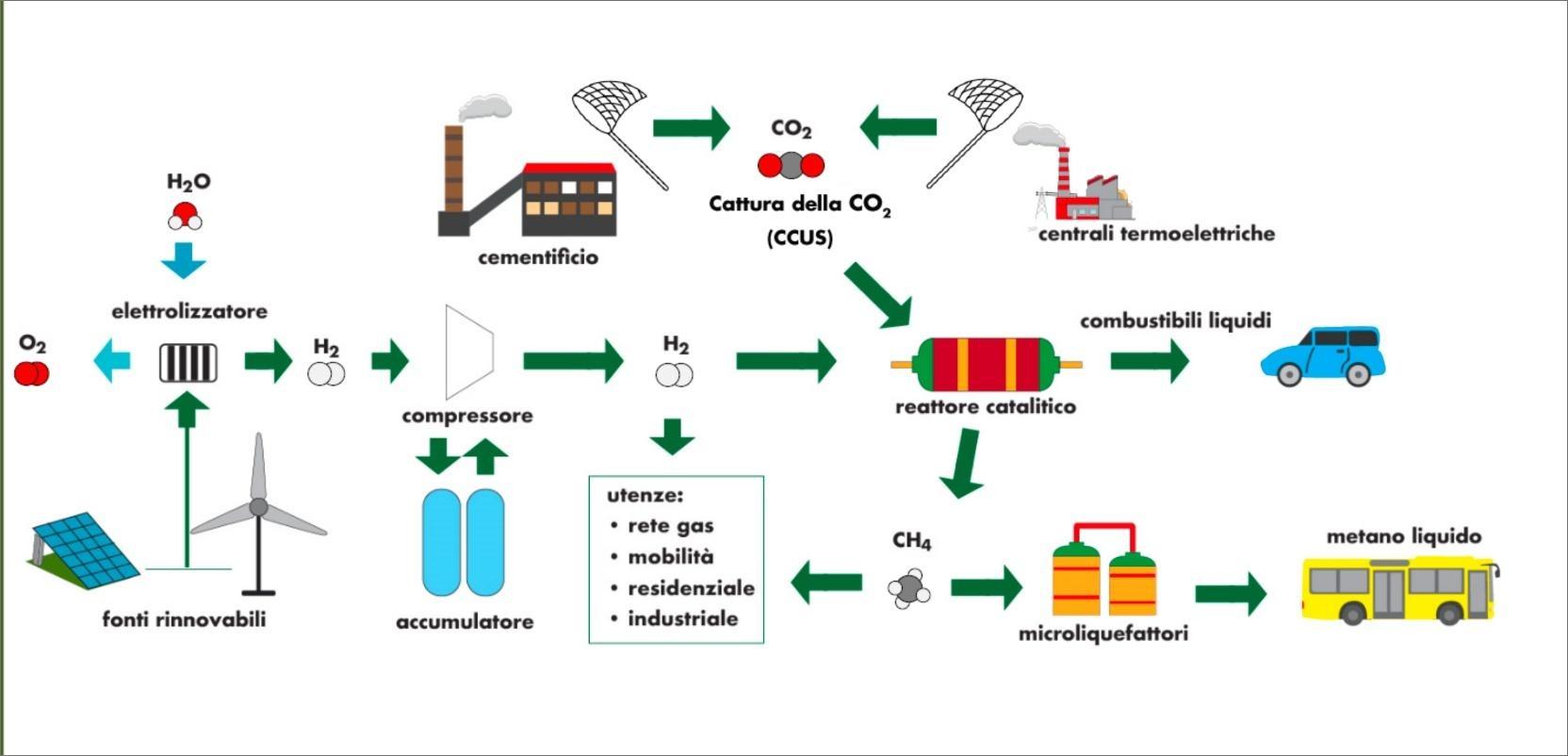
A good example is the aggregation of industries characterized by high local CO2 emissions, with others that develop technologies for the production of energy and hydrogen from renewable sources and, to close the supply chain, with the potential end users interested in e-fuels (such as the transport sector) [8][9].
Conclusions
Worldwide, private and industrial consumers are accustomed to energy prices that exclude external costs and often include subsidies. Under such conditions, renewable fuels – especially E-fuels – are unable to reach competitiveness with their non-climate-friendly competitors. Currently, one of the main challenges are relatively high production costs for E-fuels due to the lack of industrial scaling of technology, which means high investment costs. In addition, state-induced price elements can substantially increase operational costs. E-fuels are a missing link for a future low emission energy system. But although being necessary for achieving climate mitigation goals, E-fuels are facing competitive disadvantages at present. Therefore, political support is necessary for their entry into the global market. Synthetic fuels are currently inefficient in terms of energy required for production and are confronted with high production costs. Support to progress the development of this conversion technology, including demonstration and upscaling of the full production process, is relevant with a view to having substitutes for fossil fuels in particular in the most difficult to decarbonise sectors.
REFERENCES
- Prognos, SBFZ & Fraunhofer UMSICHT, Status and perspectives of liquid energy sources in the energy transitiono Title. 2018.
- Brynolf S, Taljegard M, Grahn M, Hansson J. Electrofuels for the transport sector: A review of production costs. Renew Sustain Energy Rev 2018;81:1887–905. https://doi.org/10.1016/j.rser.2017.05.288.
- Bassano C, Deiana P, Lietti L, Visconti CG. P2G movable modular plant operation on synthetic methane production from CO2 and hydrogen from renewables sources. Fuel 2019;253:1071–9. https://doi.org/10.1016/j.fuel.2019.05.074.
- https://www.powerfuels.org/powerfuels/ n.d.
- Barbarossa V, Viscardi R, Di Nardo A, Santagata A. Kinetic parameter estimation for methanol dehydration to dimethyl ether over sulfonic and polymeric acid catalysts. J Chem Technol Biotechnol 2020;95:1739–47. https://doi.org/10.1002/jctb.6372.
- Viscardi R, Barbarossa V, Gattia DM, Maggi R, Maestri G, Pancrazzi F. Effect of surface acidity on the catalytic activity and deactivation of supported sulfonic acids during dehydration of methanol to DME. New J Chem 2020;44:16810–20. https://doi.org/10.1039/D0NJ00229A.
- Viscardi R, Barbarossa V, Maggi R, Pancrazzi F. Effect of acidic MCM-41 mesoporous silica functionalized with sulfonic acid groups catalyst in conversion of methanol to dimethyl ether. Energy Reports 2020;6:49–55. https://doi.org/10.1016/j.egyr.2020.10.042.
- http://www.piugas.enea.it/; https://e-co2.it/ n.d.
- http://www.piugas.enea.it/